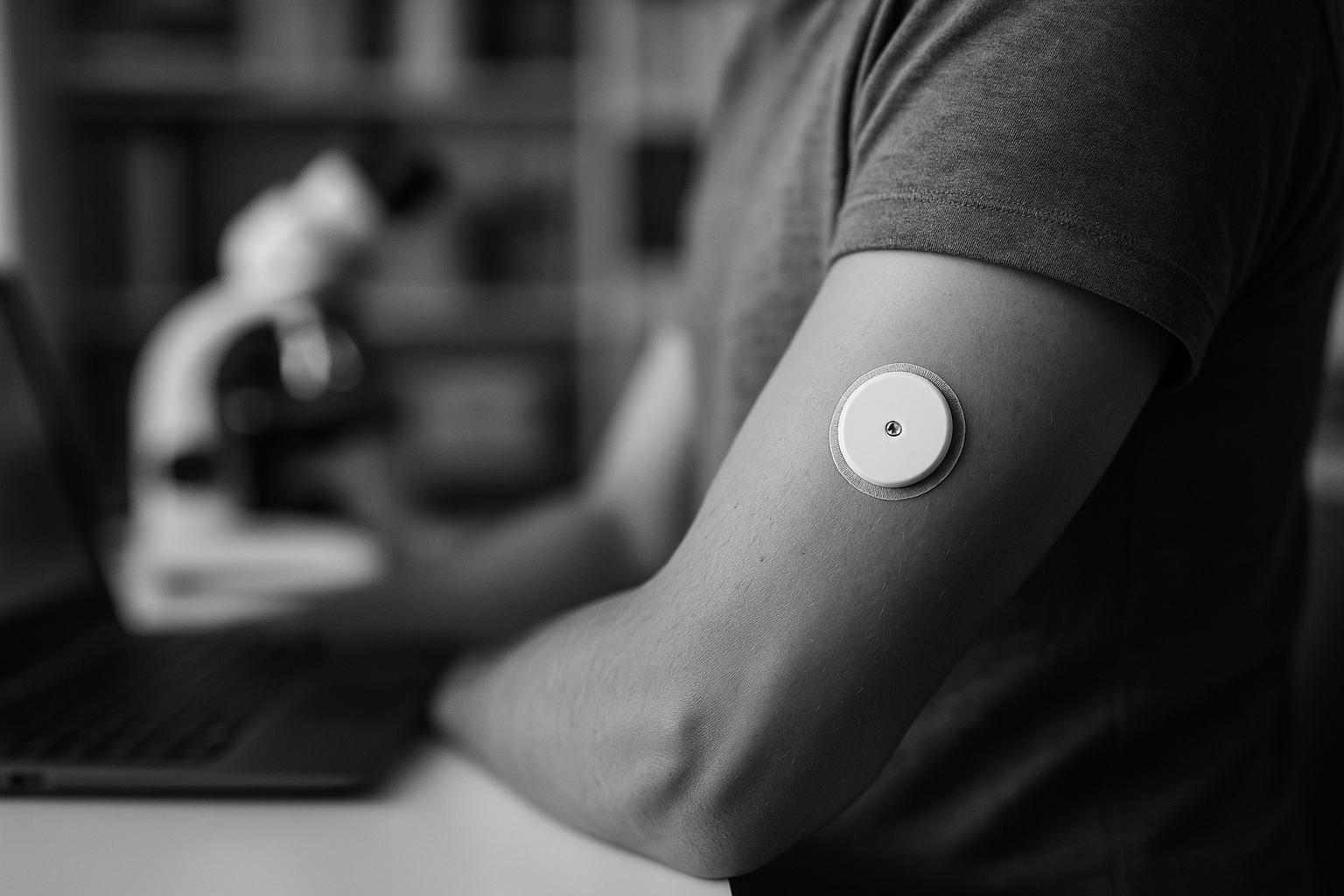
London-based health tech startup Sava Technologies has secured $19 million in Series A funding after reporting promising early results from its pre-pivotal clinical trial of a next-generation wearable biosensor. Founded in 2019 by Imperial College London bioengineers Renato Circi and Rafaël Michali, Sava is developing a proprietary multi-molecule biosensor designed to detect biomarkers just beneath the skin in real time. The device aims to offer a pain-free, affordable, and scalable alternative to current continuous glucose monitors (CGMs), which commonly face limitations around cost, invasiveness, and the duration of accurate sensor readings.
The latest clinical trial, conducted independently across sites in Oxford and Cambridge, enrolled 50 patients with Type 1 and insulin-dependent Type 2 diabetes. Early data from the first 25 participants demonstrated that Sava’s wearable delivered accurate and reliable glucose readings continuously for up to 10 days — a significant advancement compared to existing microsensor platforms, which typically struggle to maintain accuracy beyond 24 hours and rarely last more than five days. The trial was developed with input from leading diabetes clinicians and regulators, laying critical groundwork for regulatory submissions and a pivotal study planned for launch next year.
Rafaël Michali, co-founder and co-CEO of Sava, described the trial as a pivotal moment with the potential to redefine biosensing and personalised healthcare. He highlighted that their technology could match the performance of leading CGMs without the invasiveness or high costs associated with filament-based systems, opening new approaches to managing chronic diseases and more personalised health goals.
Sava’s innovation is targeted at overcoming key barriers hindering CGM adoption, including pain, expense, and limited accessibility. Currently, only about 1 percent of people with diabetes worldwide use CGMs, although this small group generates over $11 billion in annual global sales, growing at 10 percent annually. By delivering an affordable and pain-free device, Sava hopes to dramatically expand access and reshape diabetes management for millions who cannot afford or tolerate existing devices.
The $19 million Series A round was led by Balderton Capital and Pentland Ventures, with additional participation from Norrsken VC, JamJar Investments, True, Italian Founders Fund, Athletico Ventures, and Exceptional Ventures. James Wise, Partner at Balderton Capital, emphasised how Sava’s technology could democratise access not only to glucose monitoring but also to a wide range of biomarkers, expanding possibilities for preventive and personalised health management far beyond diabetes.
While glucose monitoring is Sava’s initial focus, its modular biosensor platform is designed for multi-analyte detection, enabling real-time tracking of various molecules. This versatility could attract athletes, health-conscious consumers, and others interested in optimising wellness, positioning Sava to enter the rapidly growing global wearables market, projected to exceed $100 billion by 2029. Co-founder Renato Circi noted that the platform will support multiple molecules in future iterations, creating new use cases across personalised healthcare.
Sava’s total funding now stands at $32 million, including earlier seed investments led by Balderton Capital and Exor Ventures. The seed round, announced in 2024, initially raised $8 million to develop the company’s revolutionary microneedle-based biosensor technology. This earlier funding supported foundational work on Sava’s microsensor capable of detecting molecules in interstitial fluid, as well as clinical validations and manufacturing scale-up plans.
The company has already achieved UK Medicines and Healthcare Products Regulatory Agency (MHRA) approvals for clinical trials, collaborating with prominent researchers at the Universities of Oxford and Cambridge. Beyond diabetes, Sava’s modular approach could monitor molecules like ketones, urea, proteins, allergens, or alcohol, unlocking new opportunities in chronic disease management, drug adherence, and wellbeing.
Pentland Ventures’ investor Charlie Rubin remarked that Sava, with its world-first technology and breakthrough clinical results, is well positioned to lead innovation in the multibillion-dollar global health wearables sector. The new funding will further grow Sava’s team, enhance automated manufacturing for target launch volumes, and accelerate clinical validation necessary to bring its microsensor technology to market.
As continuous glucose monitoring evolves, Sava’s platform promises a transformative step toward more accessible, painless, and personalised healthcare solutions. Its ability to extend sensor life and broaden biomarker detection highlights significant advancements over existing devices dominated by filament-based systems that remain costly and moderately invasive. If Sava successfully navigates regulatory pathways and commercial expansion, it could mark a milestone in preventative precision health, benefiting millions with diabetes and potentially revolutionising broader health monitoring.
📌 Reference Map:
- Paragraph 1 – [1], [2], [6]
- Paragraph 2 – [1], [2], [6]
- Paragraph 3 – [1]
- Paragraph 4 – [1]
- Paragraph 5 – [1]
- Paragraph 6 – [1], [6]
- Paragraph 7 – [1], [6]
- Paragraph 8 – [1], [3], [4], [5], [6], [7]
- Paragraph 9 – [1], [6]
- Paragraph 10 – [1], [6]
- Paragraph 11 – [1], [3], [4], [6]
Source: Noah Wire Services